核酸药物市场及Blockmir平台前景观察
日期:2023年6月19日 来源:财经新闻网 核酸药物市场及Blockmir平台前景观察_财经新闻网 (chnews.net)
关于小核酸药物市场前景
一、小核酸药物研发情况
人类与疾病之间的战争,与生俱来,由来已久。有没有一种“完美药物”,指哪就打哪、副作用极小……这是真实存在,抑或只是一个梦想?小核酸药物,可以说就承载着这样一个人类的“完美药物”之梦。
在人体疾病相关的致病蛋白中,超过80%的蛋白质不能被目前常规的小分子药物以及生物大分子制剂所靶向,属于不可成药蛋白质靶点。而RNA(核糖核酸)是连接基因与蛋白质的重要桥梁,因此核酸药物不仅不受自身结构制约,能够打破“难以成药性”;还能大幅度扩展靶点范围,打破“不可成药性”。传统药物受限于500个左右可成药基因,而RNA技术可围绕 2000多个已知靶基因开展制药研发。可以说,RNA技术拓宽了人类药物的来源和开发方向,理论上可以抑制任何基因。也就是说,目前尚无药物的遗传疾病和其他难治疾病,都可望通过这一途径找到解决方案。
近年来,小核酸药物因设计和开发不受限于蛋白质的可成药性及靶点的局限,被寄希望成为继小分子药物、重组蛋白/抗体药物之后的“第三次制药浪潮”。核酸药物也被公认为对抗肿瘤、老年痴呆、糖尿病等顽疾的“利器”。核酸药物的研发周期短和成功率高,也刷新了投资界对传统生物医药产业的印象。不同于小分子药物开发需要大规模化合物筛选,小核酸药物的数字化设计使其早期研发速度更快,临床研发成功率也有突破性变革。以领先药厂Alnylam为例,其研发项目从1期临床进展到3期临床开发成功率达到59.2%,远高于制药行业平均5.5%的临床开发成功率。
随着核心技术的不断突破,核酸药物逐渐发挥出了巨大的市场潜力,正在改变疾病的治疗方式。
二、小核酸药物成药上市情况
1、上市销售情况
2016年,lonis&Biogen开发的Spinraza,Sarepta Therapeutics开发的Exondys 51相继上市,拉开小核酸药物开始快速发展序幕。截至目前,全球已有16款小核酸药物获批上市(但Kynamro、Vitravene、Macugen三款早期产品已经退市),包括14款小核酸药物和2款mRNA疫苗。其中7款年销售额超过了1亿美元,尤其是Spinraza在2022年获得17.94亿美元销售额。据2023年各公司Q1财报统计,全球上市小核酸药物营收合计已达到10.218亿美元(不包括日本新药Viltepso)
市场倾向认为,ASO(反义寡核苷酸)药物商业化发展更为成熟,在整体小核酸药物中的份额达到近80%。ASO药物Spinraza是目前全世界上市的最贵的小核酸药物,作为首个获得FDA批准的SMA治疗药物,Spinraza由Biogen和Ionis合作研发,于2016年12月获得美国FDA正式批准;2017年6月在欧盟获批;2019年2月在中国上市。据美国国家生物信息中心官网显示,Spinraza在美国的标价为每次注射11.8万美元,这使得第一年的治疗成本为70.8万美元,之后每年为35.4万美元。在中国医药信息平台中显示,Spinraza注射液参考价格为69.7万一支。
此外,2023年3月7日,阿斯利康制药与Ionis公司的ASO类型药物“Eplontersen”在美国申请上市,用于治疗遗传性转甲状腺素蛋白淀粉样变性。该药于2022年初由美国食品和药物管理局(FDA)已授予孤儿药资格(ODD)。
2020年全球小核酸药物销售额为30亿美元左右,2021年全球小核酸药物销售额为32.5亿美元,小核酸药物全球市场规模从2016 年0.1 亿美元已增长至2021 年32.5 亿美元,年复合增长率高达217.8%。而2022年公开数据显示,全球15款获批上市的小核酸药物市场规模已达37.84亿美元,同比增长14%。预计到2025年全球小核酸药物销售额将突破100亿美元,复合增速达到66%。我们有理由相信,未来随着临床阶段核酸药物的不断验证与上市,将进一步驱动市场快速发展,千亿级核酸药物市场即将开启。
在我国,除Spinraza作为孤儿药获批上市外,尚无其他小核酸药物在国内获批上市。但国内也有不少企业布局,如圣诺制药、瑞博生物、悦康药业(2021年收购天龙药业, 布局核酸药物研发平台)等;相关上游企业有兆维生物、吉玛基因等,小核酸药物CDMO公司有锐博生物、凯莱英和药明康德等。业内看好的ASO药物方向,国内的代表性企业一般认为是苏州瑞博。
2、成药研发前景展望
从上市药物的适应症来看,主要集中在罕见病,但随着小核酸药物在慢病、肝炎、肿瘤等领域的应用越来越广,将在众多适应症里将拥有更大的开发潜力和临床价值;小核酸药物临床在研管线中不乏针对肿瘤、糖尿病、乙肝等大病种的临床试验, 将极大地弥补商业化重磅品种乏力的现状,潜力巨大。未来一旦在这些领域有药品成功推出,市场空间无疑将呈几何倍数放大。
小核酸药物领域的探索还仍然处于前端阶段,但小核酸药物所具有的研发周期短、效果持久、研发成功率较高、不易产生耐药性和治疗领域广等优点使得它成为医药界的新宠。

三、小核酸药物领域投融资情况
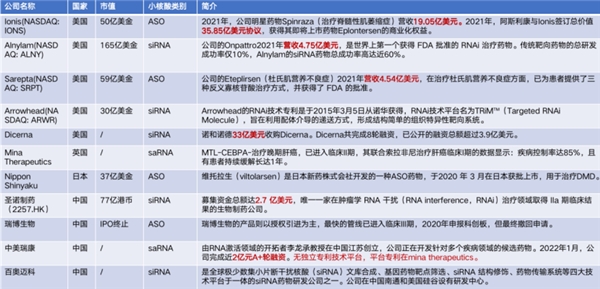
现在入局抗体药、小分子药,从美股的市值能看出一些未来的趋势,ai药学头部公司市值:17亿美金;合成生物学头部公司市值:48亿美金;核酸药物头部公司市值:270亿美金,几家规模较小的核酸企业也有90亿美金估值。舶望制药成立于2021年,2021年4月获得天使轮融资后,2022年即获得了超过4亿元的A轮融资,用于推进RNA药物进入IND。
据药融云数据库显示,2022年国内小核酸药物领域投融资事件共发生10起。
1. 中美瑞康完成近2亿元A+轮融资
融资时间:2022-01-27
投资机构: 国投创业、日本卫材、领承创投、中新资本、相和澄道
中美瑞康是一家立足于中国和面向全球市场的平台型新药研发公司,致力于开发和商业化RNA激活技术。中美瑞康已搭建了具有独立自主知识产权的“Smart-TTC”和“SCAD”两大关键技术平台,积极推进10余个小激活RNA(saRNA)药物研发管线,在罕见病、肿瘤、中枢神经系统、肝脏靶点等领域发展迅速,其中2个关键项目已进入IND-enabling开发阶段。
2. 艾码生物完成数千万元天使轮融资
融资时间: 2022-02-07
投资机构: 鼎晖投资VGC基金
艾码生物专注于临床需求尚未满足的疾病的药物开发,特别是中枢神经系统疾病、感染和肿瘤等领域的合成生物学自组装外泌体核酸类药物的开发。该公司拥有的全新的第三代小核酸递送系统,有望突破核酸药物的递送瓶颈,实现全新的多功能基因组件的靶向递送,力争打造全球领先的平台型核酸创新药公司。
3. 舶望制药完成超4亿元A轮融资
融资时间: 2022-03-23
投资机构: 正心谷资本、CPE源峰、道远资本、三一创新投资、金沙江联合创投
舶望制药成立于2021年,由数位siRNA药物开发经验丰富的科学家创立。该 公司致力于开发新一代siRNA药物,为全球患者提供更好的治疗手段。团队在核酸序列设计、化学修饰、GalNAc递送技术、肝脏外递送技术、寡聚核酸合成、CMC等RNAi药物开发的全流程环节拥有多年专业经验。
4. 大睿生物完成3300万美元 A轮 融资
融资时间: 2022-04-07
投资机构: 礼来亚洲基金、招银国际、Platanus、丰川投资、澜亭资本
大睿生物成立于2021年, 致力于发现和开发模块化和可编程的核酸药物,以惠及世界各地的患者。在蓝筹投资者的支持下,大睿生物开发了自有知识产权的 RAZORTM平台,从下一代化学修饰和新颖的生物学见解中研发和开发差异化的siRNA药物分子。
5. 瑞博生物完成4000万美元E1轮融资
融资时间:2022-07-29
投资机构: 磐霖资本、三一创新基金
瑞博生物于2007年由资深海归博士和国内专家团队创立,致力于自主知识产权的小核酸药物研发,以及在全球范围内开展临床项目研发及商业化。瑞博生物自主研发的高效长效 RIBO-GalSTARTM肝靶向递送技术达到国际水平。该公司持续发展收获了众多投资者的青睐,已完成多轮总金额超过15亿元的股权融资。
6. 昂拓生物完成近亿元种子轮融资
融资时间: 2022-09-19
投资机构: 杏泽资本
昂拓生物成立于2022年,是一家基于反义核酸技术的药物开发企业。基于团队过往在业界的前沿研究,昂拓生物搭建了国际领先的独特高效siRNA技术平台和双向调控蛋白表达的ASO技术平台,从而极大扩展了可治疗的疾病范围。昂拓生物目前着力于基于新型反义核酸技术的创新药物的研发,主要开发治疗肝靶向以及肝外靶向的代谢类常见病以及严重罕见病的核酸药物。
7. 佑嘉生物完成数千万元A+轮融资
融资时间: 2022-09-23
投资机构: 陕投成长基金
佑嘉生物是由多名海归博士和具有丰富产业化经验的团队联合创建,产品管线覆盖抗纤维化、抗病毒、心血管疾病等领域。目前已建立成熟的核酸药物全流程开发平台,包括核酸药物智能设计NDDS系统、药物评价系统、CPNPPNACLNP递送系统和核酸药物CMC平台。
8. 浩博医药完成约1600万美元Pre-A轮融资
融资时间: 2022-10-12
投资机构: InnoPinnacle Fund
浩博医药成立于2019年,致力于研发用于治疗和预防慢性乙型肝炎及其他重大传染疾病的创新药物和疫苗。 目前,浩博医药布局的三大创新管线:乙肝治愈小核酸药物平台、乙肝治疗性疫苗平台、合作开发的鼻喷抗体平台都已经进入了临床申报阶段。
9. 迦进生物完成数千万元人民币天使轮融资
融资时间: 2022-11-09
投资机构:经纬创投、峰瑞资本
迦进生物于2022年1月成立于上海张江科学城,是国内首批从事小核酸偶联药物(X-oligo conjugate,XOC)研发的生物科技公司,专注于以偶联方式实现小核酸的肝外递送,填补了国内在这一细分领域的空白。
10. 安天圣施完成2000万元pre-A轮融资
融资时间:2022-12-22
投资机构: 和瑞创投
安天圣施成立于2017年,位于苏州工业园区,公司系中国首个以RNA剪接和RNA编辑为靶标研发反义寡核苷酸(ASO)药物的公司,拥有多项国际最新ASO技术。该公司有多个项目在推进中,其中针对杜氏肌营养不良症(DMD)、 脊髓小脑性共济失调3型 (SCA3)等疾病的药物研发已取得重要进展。

总结如下:
1.相对于传统的小分子药和抗体药,核酸药物的成药性较高;在目前的技术条件下,小核酸药物可以沉默肝脏内任何基因。理论上来说,当更多的递送技术被开发出来时,小核酸药物可以靶向全基因组里的每个基因。 2. 小核酸药物半衰期比较长,从而给药周期也更长。此外,相比基因治疗,小核酸药物只影响mRNA,而不影响基因组,意味着其具有更高的安全性。 3.任何新兴技术产业都会必然经历的Gartner曲线,即发展周期必然要经历的萌芽期、过热期、低谷期、复苏期、成熟期五个阶段。阶段性的泡沫破裂不可避免,却并不是坏事,唯有如此,新兴技术才能从无序走向有序,成长为真正的长期价值创造者。中国创新药行业目前大概正处于Gartner曲线的低谷期。小核酸药物即将走完市场爆发前的最后几步,将在全球的医药产业领域迎来黄金发展期。
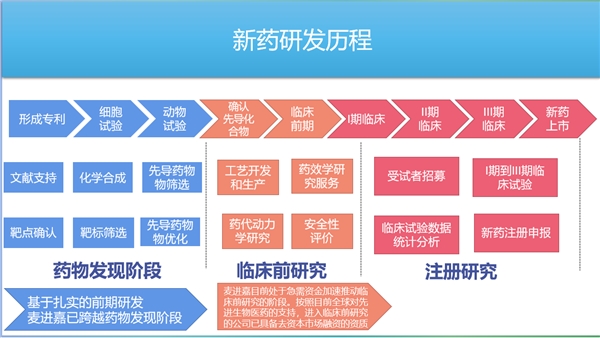
关于麦进嘉公司
第一大优势:严密而完整的专利墙
国内小核酸公司基本都是中外合资,优秀靶点的专利也基本掌握在欧美发达国家的手中,总体来看,国内小核酸赛道,正处于追赶的上升阶段。
麦进嘉也有“丹麦元素”,有锁核苷酸发明者、丹麦皇家科学院院士的支持和加盟,但与许多小核酸公司不同的是,麦进嘉公司研发的Blockmir核酸药物研发技术和Dosevo核酸药物筛选技术,公司拥有所有相关专利的全球所有权,而非一段时间或某个地域的使用权。同时,公司对Blockmir技术在全球申请广泛的专利保护,在欧洲、北美、中国和日本都拥有广泛的专利组合,涵盖整个平台(327个miRNA及其所有mRNA靶点)、第二代修饰模式,以及针对特定靶点的几个单独BlockmiR,还包含一项从带有或不带有共轭的大型文库中筛选Blockmir的方法,形成密不透风的专利保护墙。对Blockmir技术专利的完全知识产权的绝对控制,使公司牢牢掌握了主动权,这对公司下一步发展重要性不言而喻。此前,苏州一家知名小核酸药厂,也就是本文前述提及的ASO(反义核酸药物)在国内的代表厂家苏州瑞博,就因为管线来源于国外生物科技公司授权,递交上市申请时,被监管层质疑其创新能力,最终撤回了上市申请。
第二大优势:可迅速复制的平台与产品管线并重
疫情之后,biotech(生物科技)创投界最火的可能就是“平台”型公司了。一旦“平台”技术被验证,似乎就意味着公司可以大规模复制成功经验,再也不用担心管线和产品,也就不用担心上市和回报了。但是这些高估值的公司在新冠疫情后的资本寒冬中受到的冲击也最大。原因何在?光有“平台”没有“产品”是重要原因。简而言之,研发项目的质量比数量更重要。如果项目质量低下,那么平台的价值就会随着规模的扩大而降低。一个有价值的平台是能够找到少量有价值的项目,而不是大量平庸的项目。可以预期,有 first-in-class或best-in-clas类潜力的公司,一定既拥有可复制能力的平台、又有进行关键研究的在研管线、有待监管部门审批的产品等“强劲后手”。
麦进嘉公司站在Jesper Wengel院士团队15年研究成果之上,已建立起高效、紧密专利保护墙的基于Blockmir技术的药物筛选平台。多项动物试验已证实了blockmir技术的效果;完成了Blockmir小鼠脑内分布试验,确认了Blockmir药物在大脑内能到达几乎所有部位,能进入多种功能细胞;确认了Blockmir的确能在小鼠体内上调蛋白表达(Aldo A靶点);部分确认了Blockmir药物的半衰期等。2007年至今,国际上不同的课题组都对blockmir技术做过研究,他们的研究成果均被国际上的顶级期刊认可,目前国际累计发表的关的相关文献已经超过了一百多篇。
除了平台,麦进嘉在研管线多达8条,包括脑海绵体畸形、急性呼吸窘迫综合征、非酒精性脂肪性肝病、帕金森氏病、老年性黄斑病变、葡萄糖转运体1缺陷综合征、衰老、丙型肝炎等。中国科学院深圳先进技术研究院正在基于 Blockmir 技术平台与麦进嘉共同开发一种治疗罕见神经退行性疾病中的蛋白质疾病的新疗法,即将完成细胞实验,准备开展动物实验。中山大学附属第一医院也正与麦进嘉合作进行的针对急性呼吸窘迫综合征(ARDS)的核酸药物研究,已经进入了动物实验,目前已获数据正向。除此之外,平台建立了肝脏和中枢神经系统疾病的早期研究管道,并已经为这些研究管道选择了目标,在基因和疾病之间找到了直接联系。
第三大优势:逾越难关的先进技术
首先,业内公认的小核酸药物成药的最大难关就是递送技术。通俗来讲,就是药物在血液循环中容易被迅速降解,没法顺利到达细胞质中的作用位点。因此,递送系统技术是药物能否进入细胞发挥作用的关键,也是保证核酸药物的有效性和安全性的关键。也正因为此,小核酸药物的发展也很大程度上依赖递送技术的进步,纵观每次技术浪潮的兴起,递送技术都是重要的推手。
但对于麦进嘉所拥有的Blockmir平台来说,难关不一定只能攻克,还可以逾越:Blockmir技术主要锚定的是反义核酸药物,可以做到不依赖于递送系统,通过化学修饰就能保持小核酸结构稳定。反义核酸药物能做到在体外不容易降解(这点不输化学药物)、在体内不容易被RNA酶降解。所以,通过局部注射(中枢神经系统递送)就可以到达作用位点,产生足够药效。特别是Blockmir药物系统给药时还能自动靶向肝肾,因此,对于肝肾相关适应症,Blockmir药物已完全不需依赖递送系统。
这并非实验室的空想设定,目前已上市的九款ASO(反义核酸药物)都是化学修饰,并未用到递送系统。事实已证明,ASO的链条长度较短,对递送系统的依赖程度不像siRNA或者mRNA疫苗那么大。
其次,即使同为反义核酸药物,麦进嘉的技术也有关键的优胜点,即麦进嘉是上调蛋白技术,属于稀缺技术板块。中国科学院深圳先进技术研究院内尔神经可塑性诺奖实验室执行主任朱英杰对Blockmir核酸药物也特别另眼相看:“这种核酸药物很特别。大多数疾病包括肿瘤、病毒感染、免疫性疾病、代谢紊乱等,都与miRNA异常有关;目前针对miRNA的药物均抑制mRNA功能,但Blockmir能够上调mRNA的功能;一个Blockmir只上调一个mRNA功能。”这相当于其他药物是给人体做“减法”,“减”的同时难免带来副作用,而Blockmir核酸药物做的是“加法”,自然特异性更高,副作用更小。
目前市场上在研的核酸类药物大部分是通过下调mRNA的功能起作用,仅能覆盖50%的疾病;而另外50%的人类基因受 microRNA的负性调控,包括几乎所有的疾病,如肿瘤、免疫系统疾病、代谢性疾病等都因之而生,上调可以使人体被负性调控而缺失的蛋白恢复表达,因此以上大类疾病从原理上讲都能成为Blockmir技术的治疗对象,有巨大的临床需求。
第四大优势:Big pharma(知名大药企)的背书
首先陈述两个事实:
第一,麦进嘉已经与法国药企施维雅达成里程碑式的合作,这是一项预付款近200万欧元+3500万欧元合作,施维雅的反馈是专利技术初步可以成药。目前已通过施维雅的no-to-go agreement中期验收,会议纪要表明施维雅认可麦进嘉专利技术,并表示要继续持续的投入研发。施维雅目前向麦进嘉丹麦实验室提供了自己的机器作为实验器材,派驻技术人员现场协助,以加快研发速度。
生物科技初创企业有很多,但是能在初创期就和著名药企达成合作的初创企业是凤毛麟角;很多企业都是有专利免费给药厂试用,很少有大药厂愿意付费给一个名不见经传的初创企业,并且还官宣的。这意味着麦进嘉的原研技术,已经得到了法国施维雅的认可”。
大基金的背书在美国并没有太大说服力,而大药企的背书在美国却是有效的,因为真金白银是最简单直接的证据——大药企的合作,预示着极度接近管线成功成药的前景。传奇生物还未有营收的时候,被强生背书了;天境生物被艾伯维背书了,现在这两家企业都成功上市了。美股市场对biotech的判断相对理性和中立,美股只相信大药企真金白银的合作,所以,施维雅的背书已经帮我们重复验证了麦进嘉技术确实是可行的。
第二,丹麦大药厂santaris为了抢夺Blockmir专利,打了六年官司,也舍不得放手这个专利,当然官司最后以麦进嘉胜出结束。santaris是罗氏旗下收购的公司,说明麦进嘉的技术平台同时被罗氏和施维雅看上了。麦进嘉虽然只有成立三年,但由丹麦皇家科学院院士带领的团队进行的基础研究足足有15年,麦进嘉相当于凭借自己的眼光捡了个大便宜。这也说明,由于上调蛋白技术有着巨大的未被满足的临床需求,多家欧美大药企都开始下手布局上调蛋白的核酸药企。以mina therapeutics为例,这是一家英国公司,它也可以进行上调蛋白技术研发,它成立十年后才拿到第一家药企的背书,而麦进嘉成立三年就拿到了施维雅的背书,并且麦进嘉和mina的发家史特别相似——mina的创始人也是在早期眼光独到买下了所有专利开始创业,而它现在已经和大药企合作接近20亿美金了。
第五大优势:严苛实验数据管理
同样以那些从未被开发成药的陌生蛋白为靶点,为何某些临床开发就较为顺利,某些却成为前赴后继的大坑?为何有的靶点,同样的分子,在大手大脚的Big Pharma手上就做不成,在精雕细琢的Biotech创业公司上就能找到最合适的临床开发方案(适应症、目标人群、联用组合等等)?运气固然是部分原因,但是否深入探寻目标疾病的生物学机制、扎实获取真实可信全面的数据,恐怕才是关键的胜负手。有专家接受采访时也提到这个现象:“临床前工作没有仔细去做,就想直接上临床。一上临床,爬到比较高的剂量,发现没效果。至于为什么没效果呢?靶点还没完全搞清楚。”
麦进嘉公司对数据要求极其严苛,与市面上一些公司用AI“做”数据或仅以一两例阳性数据以偏概全不同,创始人坚守科学精神,避免因为假阳性数据造成产业化实施失败。这个理念甚至能够给予国内创新药投资纠结的“投早投小”还是“投后期”提供一些启示。如果既想规避内卷,挖掘一个独特的创新靶点项目,又希望尽量减少风险,有足够的确定性,那么已经进入研发后期、数据更为充分的这类项目,相较而言更不错的选择,尤其当研发公司既有足够积极的临床前数据,又有自己对目标靶点比较深入且独特的疾病生物学机制相关转化医学研究。
第六大优势:成熟的产业化经验
长期以来,中小型Biotech公司是推动小核酸药物技术进步的主要动力。随着产品在临床开发中取得进展,价值呈指数增长。数以百计的研发阶段的项目的价值,不及一个获得批准的重磅炸弹产品。
但也必须看到,创新药研发本身就是富贵险中求的生意,除了承担研发的科学风险,还要面对产业化的商业风险。首先,要从药理、毒理、PK/PD出发,然后寻找动物和人之间的这个 gap ,并且推断这些gap未来在临床中所带来的影响;其次,需要了解药政部门对这些gap的看法,一方面需要了解临床研究者处理这些gap的方法,还要兼顾到企业的利益,做到企业利益最大化。选择好时机、围绕一个有管线和产品候选的平台来筹集种子资金或A轮融资无疑是有效降低风险的方法。在2010-2022年间上市的500多家生物技术初创企业中,有32家以超过10亿美元的价格被收购。所有这些公司都有降低风险的产品——40% 已经有产品获得批准,31% 已经提交或即将提交监管文件以获得批准,25% 正在进行关键性研究。
而麦进嘉正好是一家拥有产业化经验的创新型Biotech公司。公司创始人翟嘉洁博士同时也是另一家医疗器械公司——佳悦美视生物科技术有限公司创始人。佳悦美视公司于2016年11月成立,是一家专注于研究和生产眼科器械的科技型企业,获得 了广州开发区产业基金的投资。公司研制的领扣型人工角膜2021年4月6日入选第一批创新服务重点目录“创新医 疗器械项目”,2021年2月因“临床急需,且在我国尚无同品种产品获准注册”,获得国家药品监督管理局医疗 器械技术审评中心予以优先审评,2021年9月取得III类医疗器械注册证,2021年10月获得III类医疗器械生产许可证。目前领扣型人工角膜复明手术已成为中山大学眼科医院、解放军301医院、山东省眼科医院等国内多家知名眼科医院或综合医院眼科常规手术。
因为有丰富的产业化经验,麦进嘉才有底气承诺:在本次投资方全部投资价款到位之日起至 2026 年 03 月 11 日,实现系统的、 可再生产的、可拓展的 Blockmir 专利技术; 在本次投资方全部投资价款到位之日起至 2026 年 03 月 11 日,至少 1 个靶点 完成既定治疗目标,实现可商业化;可商业化具体指自有研发产品管线独家授权许可给其他药企进行全球或部分地区范围内的研发、生产和商业化销售,或与其他药企合作开发,授权和/或合作协议约定首付款、里程碑付款、以及双方的潜在销售比例提成等;或自有研发管线整体出售获得一次性收益。 在本次投资方全部投资价款到位之日起至 2026 年 03 月 11 日,开发出一个新 药化合物进入临床前动物实验; 在本次投资方全部投资价款到位之日起至 2026 年 03 月 11 日,公司至少有一 条管线获得新药临床试验申请的批件。
综上所述,核酸药物赛道确实是未来长坡厚雪的好赛道。麦进嘉公司正处在已经被国外验证、头部企业达到200亿美金估值且国内竞争还是蓝海的核酸药物细分优质赛道上。在如此优质的赛道上,我们不应该放弃一家确定性如此之高的start-up企业。而在投资时机的选择上,显然早期(临床阶段或临床前阶段)投资最能接近红利可观和风险可控的平衡点。
